Contents
GENERAL FAQs
1. What is the difference between Citriodiol®, “Eucalyptus citriodora oil, hydrated, cyclized” (EC Oil H/C), “Oil of Lemon Eucalyptus” (OLE) and “a mixture of cis- and trans-p-menthane-3,8-diol/Citriodiol” (PMDRBO)?
Citriodiol® is Citrefine’s trade name for the generic active ingredient, referred to in Europe as Eucalyptus citriodora oil, hydrated, cyclized” (EC Oil H/C) and in the US and other jurisdictions as “Oil of Lemon Eucalyptus” (OLE) or ““Oil of Lemon Eucalyptus, hydrated, cyclized” (OLE H/C). This same active ingredient (also sometimes called PMDRBO (PMD Rich Botanic Oil)) was originally listed with the European authorities as “a mixture of cis- and trans-p-menthane-3,8-diol/Citriodiol”, but has since been redefined as Eucalyptus citriodora oil, hydrated, cyclized” (EC Oil H/C) to better reflect its botanic origin. Citriodiol® is a naturally sourced active substance containing p-menthane-3,8-diol (PMD) and other naturally occurring components from the Eucalyptus citriodora tree’s oil (common name: Lemon eucalyptus).
Please contact us to understand how to reference Citriodiol® on your labels, or if you have any further questions about this.
2. Is Citriodiol® the same as Citronella?
No, while Citriodiol® is made from an essential oil, it is very different from citronella oil. Firstly, citronella oil is obtained from the leaves and stems of different species of Cymbopogon, or lemongrass, whereas the oil for Citriodiol® is obtained from the Eucalyptus citriodora tree.
The other fundamental and biggest difference is that Citriodiol® contains at least 64% (and more typically about 72%) of the naturally occurring constituent PMD (p-menthane-3,8-diol) whereas Citronella oil contains only trace quantities of PMD. This is an important distinction because PMD is the constituent primarily responsible for the efficacy of Citriodiol® in repelling biting insects and other arthropods. In side-by-side independent comparisons, Citriodiol® products repel biting insects, such as mosquitoes, 4 to 8 times longer than citronella oil products (Barnard 2004).
Citronella can no longer be marketed as an insect repellent active ingredient in the EU, as it is not being supported under the EU Biocidal Products Regulations (BPR), whereas Citriodiol® is being supported under the BPR.
3. Is naturally sourced PMD the same as synthetic PMD?
No. The PMD in Citriodiol® is sourced directly from essential oils. The PMD created from this oil sits along-side the other naturally occurring constituents in a product with 100% purity. This naturally sourced PMD product, which is sometimes called PMD rich botanic oil (PMDRBO) or Oil of Lemon Eucalyptus (OLE) has been notified under the BPR as “Eucalyptus citriodora oil, hydrated, cyclized” and is about 50% more effective in repelling insects than like quantities of synthetic PMD.
By comparison, what is commonly referred to as synthetic PMD is not derived from an essential oil, but rather is typically is a product of far more complicated multi-step chemical processes, for example making menthol. The resulting material contains only PMD and has no essential oil co-constituents.
4. Why is naturally sourced PMD better than synthetic PMD?
Simply put, in our view it is better for the consumer and better for the environment. Consumers benefit because naturally sourced PMD (i.e. Citriodiol®) performs significantly better than synthetic PMD in efficacy tests. An independent study (Drapeau 2011) published comparing synthetic PMD with PMDRBO shows that with like amounts of PMD, it performed 50% better in repelling the target organism (in that case Aedes mosquitoes). In this study a 20% PMDRBO (which at a min 64% PMD contained ca. 13% PMD) alcohol spray offered over 5 hours complete protection against Aedes mosquitoes whereas the 13% synthetic PMD product provided only 3¼ hours of protection.
While both forms of PMD are rapidly biodegradable, the real benefit to the environment from Citriodiol® is that it actually exists in nature in its final form. This means nothing is being added to the environment that has the potential to upset its natural order and balance. In addition, because the material used to make Citriodiol® is Eucalyptus citriodora oil, and this oil is distilled just from the leaves and twigs of the tree, allowing the tree to continue growing, using Citriodiol® actually encourages the growth and maintenance of healthy trees.
5. Why was Citriodiol® notified under the CAS number for PMD if it is being treated as a pure substance?
The history of the registration of Citriodiol® in Europe has been complex, in part because it has forged new ground for the approval of botanicals as biocides. One complication has been how to identify naturally sourced PMD e.g. Citriodiol®. At the time of its notification and in the absence of a CAS Registry Number specific to Citriodiol®, the Commission advised that its main constituent in Citriodiol® is PMD, and it should be notified using the CAS RN for pure PMD (42822-86-6). We have since applied for, and been given, a unique CAS RN for Citriodiol®, which applies to the substance as a whole. This CAS RN is 1245629-80-4 and will be the number under which it is formally listed following an inclusion decision.
As of April 2017, the Art 95 list of approved suppliers includes the active substance EC Oil (H/C) but also ties this to prior name PMD. It may be helpful for customers who are working with Member State authorities unfamiliar with this change, to direct them to this listing. We now recommend notifying/registering products in Europe under the new CAS RN 1245629-80-4, since this is a substance readily found on the Art 95 list of supported substances. It is also appropriate to register products containing Citriodiol® in Canada, the USA and Australia under the new CAS number. Please check with us for products in other countries.
6. How is the Eucalyptus citriodora oil extracted?
The oil is extracted from the leaves and twigs of the Eucalyptus citriodora tree by steam distillation. You can learn more about this process by following this link.
7. How is Citriodiol® made?
As the essential oil in the leaves of the Eucalyptus citriodora tree matures, the main constituent of the oil, citronellal, gradually converts into PMD. Indeed, what we know as Citriodiol® (i.e. a high PMD content oil with minor isopulegol, citronellol and acetal co-constituents) is merely an acceleration of nature’s process.
However, steam distillation is required to extract the oil. Because PMD crystallises, distillation of oil containing PMD is not feasible. We therefore harvest the leaves at a younger age when there is only a very small amount of PMD present and the citronellal content is highest. We then use a dilute aqueous catalyst to convert the citronellal content to PMD, with small amounts of isopulegol and acetal as by-products. Finally, we wash out the catalyst. The result is a product that contains a minimum of 64% PMD and only a negligible amount of citronellal. In this way we are effectively mimicking nature’s own aging process. The other components in the Eucalyptus citriodora oil remain unaffected by process and are found in the same relative quantities in Citriodiol®.
EFFICACY FAQs
1. How does Citriodiol® work? What is its mode of action?
Female mosquitoes and other biting insects are attracted to humans and other animals by a combination of CO2, heat and odour. While the mode of action of insect repellents generally is not well understood, research indicates that repellents disrupt the odour receptors on biting insects and prevent them from landing on the skin.
2. What insects is it effective against?
Studies have shown Citriodiol® to be effective against the following species of insect and parasites:
- Mosquitoes
- Ticks
- Midges
- Horse Flies
- Sand Flies
- Head Lice
- Cat Fleas
For more information about the species and the relevant viruses and diseases, please click here.
3. Is it effective against leeches?
Citriodiol® is very effective in repelling land leeches. There is published data to support this and which can be made available to support a product claim. We do not have data on water leeches.
We are keen to understand and promote the repellence properties of Citriodiol® so if you decide to conduct efficacy testing of your own, we’d welcome the opportunity to review your results or even to comment on the proposed study protocol in advance.
4. Can it be used on animals such as horses?
As part of our development programme, Citrefine has begun to generate efficacy data for use of Citriodiol® based products as a repellent on horses. The results are promising and we will be continuing this work.
We have not yet prepared internal risk assessments as to the safe use of Citriodiol® on horses; however, it has been used at low concentrations on horses in Europe for at least a decade. In addition, our consulting toxicologist with full access to our acute toxicity, repeat dose toxicity and teratogenicity (developmental) studies is of the professional opinion that we can readily support a bridging argument and risk assessment for higher concentration formulations applied to horses.
If you are interested in more information on horses or in a product for other animals please contact us.
5. How else can Citriodiol® be used?
The data on Citriodiol® supports the use of the active substance against a range of biting insects and other arthropods in a dermally applied personal repellent. Citriodiol® is used in other applications, such as spatial repellents (candles, diffusers, etc.), application to horses to repel insects or to human hair to repel lice. While these specific uses are not included in the EU dossier, US EPA or Canadian PMRA registrations, customers are able to support these uses by developing appropriate risk assessments and efficacy data for inclusion within their product authorisation dossiers or national registrations.
If you have questions about how to use Citriodiol® in other applications please contact us.
6. Is it as effective as DEET?
CoComparing PMD content to DEET content in similar concentrations, independent entomologists have stated the efficacy of these substances is reasonably similar. See, for example, Carroll 2006, Trigg 1994. Our own internal testing shows efficacy results to be similar where comparable amounts of DEET and PMD are contained in the product. This is supported by a recent independent study by the US Consumer Reports testing the Citriodiol®-based product, Repel Lemon Eucalyptus and finding 30% formulations on par with DEET at between 7 and 8 hours of efficacy against Aedes and Culex mosquitoes and deer ticks.
7. Is efficacy related to PMD content?
Yes, the PMD content is the principle factor in predicting the efficacy of a product. It evaporates slowly from the skin, and it is while it is in this vapour phase that it has a repellent effect.
It is worth noting that PMD, when applied in conjunction with the other naturally occurring constituents in the starting material (Eucalyptus citriodora), is substantially more repellent to biting insects than pure PMD. Therefore it is more accurate to say that it is the Citriodiol® content of a product that determines efficacy.
8. What % active do you need to claim how many hours of efficacy?
Efficacy is impacted to a reasonable degree by the co-formulants in the product. For example, lotions tend to have a slightly improved efficacy over a spray, and sprays incorporating a co-formulant that acts to slow down the rate of evaporation of the PMD (a fixative) may have longer protection times over those without. In addition, efficacy times are affected by application rates, environmental conditions such as biting pressure, and by the target species.
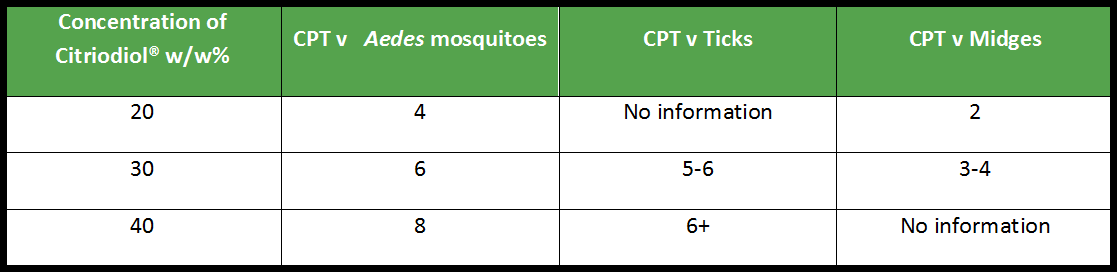
With this said, if we look at the simplest formulations applied in field conditions, you might expect to be able to support a claim of complete protection for 6 hours with a 30% product (or up to 8 hours complete protection if the authority will allow this phrasing).
9. Can it be used as a spatial repellent or in other products not applied to the skin?
In theory, yes, but it will depend on the delivery system.
We have a large body of data supporting its safety as well as its efficacy. However, the efficacy data we have is based on dermally applied products, mainly for use on humans and not spatial repellency. While it may be possible to produce a spatial repellent product, we are not able to advise on the percentage of Citriodiol® required, or its efficacy.
10. Can it be impregnated in material?
Citriodiol® has been successfully micro-encapsulated by several different companies and in this form can be used to impregnate fabric and clothing. Please contact us for further information.
PRODUCT USAGE AND SAFETY FAQs
1. Is it safe to use?
Extensive testing has shown that Citriodiol® has a low toxicity profile. As a product traditionally applied to the skin, it is particularly important that it has a low rate of dermal absorption, is not a skin sensitiser and is not classified as a dermal irritant. It is, however, a category 2 eye irritant, and formulations with a Citriodiol® content of 10% or greater will also be classified as eye irritants without specific data to the contrary. Product labels should be clear that application should not encourage spraying on or around the eyes. Citirodiol® does contain some natural allergens that have the potential to cause an allergic reaction in previously sensitised individuals, this needs to be reflected on the product label even though Citriodiol® is not classified as a sensitiser.
2. Is it safe to use on children?
Yes. Our quantitative human health risk assessment supports application to children from the age of 3 months. However, because of the very thin epidermis of children this age, and the often increased sensitivity of skin, we recommend application from 6 months. Please note, however, that requirements may vary by country so please do advise us of your specific requirements and we can provide more tailored advice.
3. Is it safe for pregnant women?
Yes. While we have not conducted studies directly on pregnant women for ethical reasons, data generated from a multi-generation toxicity studies, together with the very low rate of dermal absorption of Citriodiol®, supports unrestricted use by pregnant women. This conclusion was supported by the US EPA when it registered the product more than a decade ago allowing its unrestricted use by pregnant women when used in accordance with the product label.
4. Is it safe for the environment?
Yes, Citriodiol® is a ready biodegradable material, which means that once it leaves the skin or is disposed of and enters the waterways and STPs, it will break down very quickly. Because of this rapid degradation, there is no opportunity for any significant amounts to build up in the soil, water or air and alter the otherwise existing ecosystem.
5. Can Citriodiol® be used in products to support anti-viral claims?
No, while there is recent research by the UK government showing Citriodiol® does have anti-viral activity [Dstl study], this research is preliminary in nature. No anti-viral claim can be made about Citriodiol® products; it is only supported for use as an insect repellent. Because of the press coverage surrounding the government’s publication of these trial results, we strongly encourage our customers to make this clear to any consumers contacting them about use of Citriodiol® products.
6. Can it be applied with sunscreen?
While we have not conducted any tests to prove the safety of Citriodiol® when applied with sunscreen products, we are not aware of any evidence that would prevent their use together where it is appropriate to do so (e.g. for repelling day-biting mosquitoes in tropical climates) and the labelling requirements of both products are followed.
As Citriodiol® has a very low rate of dermal absorption and must evaporate from the skin to be effective it should be applied after a sunscreen.
7. Can it be applied under clothing?
The US Centers for Disease Control and Prevention (CDC) recommends that insect repellents are not applied under clothing. This is because there is an increase in the rate of dermal absorption due to perspiration.
8. Can it be used safely in an aerosol?
Yes. Whilst Citrefine is not currently planning to support an aerosol product within the Biocidal Product Family, the inhalation toxicity of an aerosol product containing 50% Citriodiol® (with an average particle size of <50 µm (MMAD) has been tested for inhalation toxicity and the result for this product formulation was LC50 >2.06 mg/L (rat). We would suggest that the safety of other aerosol pYes. Whilst Citrefine is not currently planning to support an aerosol product within the Biocidal Product Family, the inhalation toxicity of an aerosol product containing 50% Citriodiol® (with an average particle size of <50 µm (MMAD) has been tested for inhalation toxicity and the result for this product formulation was LC50 >2.06 mg/L (rat). We would suggest that the safety of other aerosol products containing Citriodiol® could be assessed on their similarity to the 50% Citriodiol® formulation tested for acute inhalation toxicity considering the particle size generated by the actuator used on the aerosol product. Citrefine are considering including the use of a Bag on Valve (BoV) delivery system in the Product Family that will be submitted under the BPR. The use of BoV over conventional aerosols brings several benefits such as exclusion of the use of extremely flammable gases, better product evacuation (so less product remains in can at end of life), no consumer contact with propellant and reduced use of VOCs, which is better for the environment.
REGULATORY FAQs
1. Is Citriodiol® registered under the BPR in Europe?
Citriodiol® is being supported by Citrefine for use under Product Type 19 (repellents and attractants) under the BPR. It was originally notified under the BPD/R as “a mixture of cis- and trans-p-menthane-3,8-diol/citriodiol” under CAS No. 42822-86-6. As of October 2016, it has been redefined as “Eucalyptus citriodora oil, hydrated, cyclized” under CAS No. 1245629-80-4 (abbreviated for informal use as EC Oil (H/C)) to better reflect its botanic origin. If you have any questions regarding this change and how it will affect you, please contact us directly.
Through our Irish entity, Citrefine EU Ltd is an Article 95 approved supplier of Citriodiol®. We anticipate a published inclusion decision by approximately May 2026 , which would mean Citriodiol®-based product authorisation dossiers would have a deadline for submission of approx. November 2027. Needless to see these dates are our best estimate only as the speed of the review is in the hands of the authorities.
2. Can I still place a product on the market in Europe?
Because Citriodiol® is still going through the BPR evaluation process, you are free to place a Citriodiol® product for use as a repellent on the market by obtaining a national registration as required in your territory. At the point of Union listing (expected November 2027) you will need to have submitted your Product Authorisation (PA) dossier in line with the BPR requirements, or rely on Citrefine’s submission of its Biocidal Product Family (BPF). It is critical to have your product on the market by the date of Union listing in order to continue selling it while your PA dossier or our BPF are being considered (this product evaluation takes at least 3 years). Our best estimate of the earliest date for a published inclusion decision of Citriodiol® is May 2026 and Union listing expected November 2027. Note that authorities may stop accepting new national registrations up to 18 months prior to formal Union listing, depending on Member State (MS). This is dependent on the length of time each MS product registration process takes in that country. Therefore, please ensure that all your products are registered in each target country before the start of the market freeze period. Failure to do this will mean you cannot put a new product on the market for several years (3 years minimum). Contact us for more information if you are unsure about how this will affect you. Please see the latest BPR timeline for Citriodiol® by clicking here.
3. How do I gain access to data to support product registrations?
If you have your own formulation, you will almost certainly need to develop your own phys/chem and efficacy data to support your product submission. You may be able to rely on Citrefine data to support the toxicity endpoints required. If you wish to use one of Citrefine’s formulations, we can assist further as we have a large body of data to support such product registrations. Relying on our data If you have your own formulation, you will almost certainly need to develop your own phys/chem and efficacy data to support your product submission. You may be able to rely on Citrefine’s data to support the toxicity endpoints required. If you wish to use one of Citrefine’s formulations, we can assist further as we have a large body of data to support such product registrations. Relying on our data typically involves our providing you with citation rights to certain studies through a Letter of Access (LoA). Depending on the access required, we may ask you to sign an agreement and there may also be a data access fee. Please contact us for more information about this.
4. How do I register my products?
Most jurisdictions require some sort of registration to sell insect repellent products. However, this can vary from a simple notification, to a much more complicated authorisation as a biocide or bio-pesticide. You will need to find out which authority is responsible for regulating repellents in the market of you wish to enter (an answer that may well be driven by the claims you wish to make on your product label). You should then contact the relevant authority to determine specifically what data and fees are required to make a submission, and what is the timeline for the evaluation. If there are any gaps in your own data set or data we can provide, it may be a good idea to discuss these points directly with the authority. Our regulatory team is also here to assist you in this process. If you require significant support, we would suggest that you retain the services of a specialist regulatory consultant.
5. What is the CAS No. for Citriodiol®?
In 2011 the American Chemical Society assigned the substance described as “oils, eucalyptus, E. citriodora, hydrated, cyclized” the CAS No. 1245629-80-4.
Europe
When Citriodiol® was first notified under the European BPD in 2001, the CAS No. above did not exist and so it was notified more generally as “a mixture of cis- and trans-p-menthane-3,8-diol/citriodiol” relying on the CAS No. 42822-86-6. As of October 2016, however, ECHA redefined the substance Citriodiol® to better reflect its botanic origin. It is now properly referenced for regulatory purposes as “Eucalyptus citriodora oil, hydrated, cyclized” (abbreviated for informal use as EC Oil (H/C)) under CAS No. 1245629-80-4.
Rest of the World
Citriodiol® is registered as an active ingredient for use in insect repellents under the CAS No. 1245629-80-4 in most jurisdictions, including with the EPA (USA), PMRA (Canada) and APVMA (Australia). There are a few jurisdiction in which its original CAS No. 42822-86-6 is still recognised, so if you are in doubt, please check with us before making your submission.
6. Which products/formulations will be registered under the BPR and what data supports this?
Citrefine is committed to supporting its reference product (an alcohol-based spray containing 30% Citriodiol®) as well as a 30% Citriodiol® roll-on product (a lotion) and a newly developed 20% emulsion product through Product Authorisation under the BPR. We intend to register these via a Biocidal Product Family. This may also include a 10-15% sprayable emulsion product and/or a 40% alcohol based spray, depending on customer interest.
Much of the phys/chem dataset including stability data has been developed and additional data is currently being generated on these products where data gaps exist. Analytical methods to measure the amount of PMD have been developed for these products.
7. Where else is it registered? Which product types are registered?
Citrefine has a number of formulations registered globally, including in countries such as the USA, Canada and Australia.
USA: Citriodiol® is registered with the US EPA as an active ingredient to be used in dermally applied repellents. In addition, Citrefine has both a 30% spray and a 30% lotion registered with the EPA, which include both mosquito and tick claims and no age restriction.
Canada: Citriodiol® is registered with Health Canada’s PMRA as an active ingredient to be used in dermally applied repellents. In addition, Citrefine has a 30% spray registered with the PMRA with both mosquito and tick claims.
Australia: Citriodiol® is registered with Austalia’s APVMA as an active ingredient to be used in dermally applied repellents. In addition, Citrefine has both a 30% spray and 30% roll-on registered with APVMA, which include mosquito, tick and midge claims.
Southeast Asia: Citriodiol® as a naturally sourced active ingredient, and repellents containing Citriodiol®, do not require registration in Thailand, Malaysia and Singapore. In other jurisdictions including the Philippines and Indonesia, registration is required and Citrefine is engaged in the registration process.
Please let us know the territories and product types you are interested in and we can provide further information to support the regulatory process.
TECHNICAL FAQs
1. What are the characteristics of Citriodiol®?
At room temperature it is a yellow liquid containing white crystals. When heated to 55°C to 60°C the crystals will melt to become homogeneous and will be a yellow liquid. It has a strong lemon scent.
2. How do I formulate using Citriodiol®?
We can provide you with guidance documents on how to make product formulations with Citriodiol®. Please contact us
3. Do you have “off-the-shelf” formulations I can use?
Yes. Part of the service we provide is the use of our off-the-shelf formulations using Citriodiol®. Please let us know if you are interested in learning more about this.
4. Does Citrefine have methods of analysing Citriodiol®?
Yes we have developed analytical methods for the measurement of relevant components in both Citriodiol® and our off-the-shelf formulations to share with customers.
5. What about the handling and storage of Citriodiol®?
As Citriodiol® is a semi-solid it will require homogenisation before use. This can be achieved by warming the product with stirring until no crystals are visible and the Citriodiol® is clear. Citriodiol® is provided in open top drums to facilitate this process.
Citriodiol® should be stored in the original container, tightly closed in a dry, cool and well ventilated place.
MARKETING FAQs
1. What insect bites does Citriodiol® protect against?
Citriodiol® is effective against disease carrying insects such as Mosquitoes, Ticks, Sand Flies, Black Flies and Stable Flies. It also repels annoying insects such as Midges and Land Leeches which can lead to infected insect bites.
2. Can I call it “natural”?
Citriodiol® is a naturally derived substance and only undergoes a single simple process during production. There are restrictions on the use of the word “Natural” in certain jurisdictions as some regulatory authorities believe that the use of this word conveys a meaning of safety to consumers. In those jurisdictions, using the term “natural” on the label is not permitted. However, there are very similar words and phrases which can be used such as “Botanical”, “Lemon Eucalyptus” and “Plant-based” which can convey the real environmental benefits of this active while complying with regulations.
Citriodiol® has been certified by ECOCERT as raw material that meets The COSMOS Standards for organic and natural ingredients. As a COSMOS approved ingredient, Citriodiol® can be used in end-use products which are to be certified as COSMOS natural or COSMOS organic.
The ECOCERT approval is only given to raw materials that meet the strict requirements regarding the origin of the material (e.g. not using GMOs, no endangered species). The material must also meet strict criteria relating to processing of the material (e.g. use of starting materials with strict toxicity and biodegradability limits).
3. Is it environmentally friendly?
In order to harvest the leaves of the citriodora trees for steam distillation, the trees are coppiced. This means that just the younger leaves and small branches are cut back for production, and the trunk and larger branches continue to grow. Hence the process of harvesting is sustainable. In addition, the by-product of the steam distillation which is used to draw the oil from the leaves and twigs is spread back onto the trees as a fertilizer to encourage future growth. To understand more about our process please click here.
4. Can I use the Citriodiol® logo on my products?
We encourage our customers to use the trademark “Citriodiol®” in connection with the marketing and sale of consumer goods which contain Citriodiol® as the sole active substance. We do have guidelines for the use of our trademark however, so please let us know if you wish to use our trademark and we can send these to you
Scroll to top

